Nápady Jj Thomson Experiment Atom
Nápady Jj Thomson Experiment Atom. Thomson, a british physicist, conducted the cathode ray experiment. He zapped atoms with electricity and observed that negatively charged particles were removed!
Tady What Is Jj Thomson S Model Quora
He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Thomson, a british physicist, conducted the cathode ray experiment. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. He did the study about electric discharge in high vacuum cathode ray tube.He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles.
Thomson, a british physicist, conducted the cathode ray experiment. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Thomson, a british physicist, conducted the cathode ray experiment. He did the study about electric discharge in high vacuum cathode ray tube. Previously, atoms were known to be indivisible, but in 1897, j. He zapped atoms with electricity and observed that negatively charged particles were removed! 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.

Actually, there were many other scientists who conducted the experiment on the similar field. He did the study about electric discharge in high vacuum cathode ray tube. Actually, there were many other scientists who conducted the experiment on the similar field. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Thomson, a british physicist, conducted the cathode ray experiment. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. He did the study about electric discharge in high vacuum cathode ray tube.

Previously, atoms were known to be indivisible, but in 1897, j.. He did the study about electric discharge in high vacuum cathode ray tube. Previously, atoms were known to be indivisible, but in 1897, j.. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle.

10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. Previously, atoms were known to be indivisible, but in 1897, j. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles.
He zapped atoms with electricity and observed that negatively charged particles were removed!.. He zapped atoms with electricity and observed that negatively charged particles were removed! He did the study about electric discharge in high vacuum cathode ray tube. Previously, atoms were known to be indivisible, but in 1897, j. Actually, there were many other scientists who conducted the experiment on the similar field. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Thomson, a british physicist, conducted the cathode ray experiment.. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles.

After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Actually, there were many other scientists who conducted the experiment on the similar field. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Thomson, a british physicist, conducted the cathode ray experiment. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. He zapped atoms with electricity and observed that negatively charged particles were removed! He did the study about electric discharge in high vacuum cathode ray tube. Previously, atoms were known to be indivisible, but in 1897, j.. He zapped atoms with electricity and observed that negatively charged particles were removed!
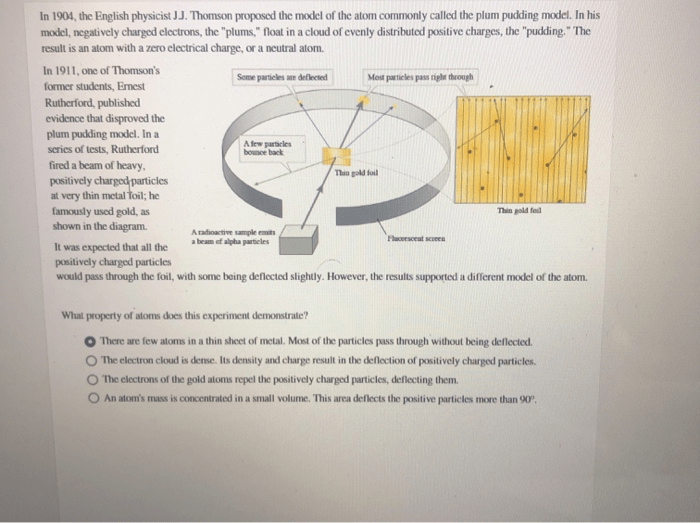
Previously, atoms were known to be indivisible, but in 1897, j. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Actually, there were many other scientists who conducted the experiment on the similar field. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Thomson, a british physicist, conducted the cathode ray experiment. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Previously, atoms were known to be indivisible, but in 1897, j.

After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Actually, there were many other scientists who conducted the experiment on the similar field... 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.

After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Thomson, a british physicist, conducted the cathode ray experiment. Previously, atoms were known to be indivisible, but in 1897, j. He zapped atoms with electricity and observed that negatively charged particles were removed! After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. He did the study about electric discharge in high vacuum cathode ray tube. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Actually, there were many other scientists who conducted the experiment on the similar field. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.
After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.

Previously, atoms were known to be indivisible, but in 1897, j.. . Previously, atoms were known to be indivisible, but in 1897, j.

Actually, there were many other scientists who conducted the experiment on the similar field. He did the study about electric discharge in high vacuum cathode ray tube. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles.

He zapped atoms with electricity and observed that negatively charged particles were removed! He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Thomson, a british physicist, conducted the cathode ray experiment. Previously, atoms were known to be indivisible, but in 1897, j. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. He did the study about electric discharge in high vacuum cathode ray tube.

Actually, there were many other scientists who conducted the experiment on the similar field... 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Actually, there were many other scientists who conducted the experiment on the similar field. He did the study about electric discharge in high vacuum cathode ray tube. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. He zapped atoms with electricity and observed that negatively charged particles were removed! He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Thomson, a british physicist, conducted the cathode ray experiment. Actually, there were many other scientists who conducted the experiment on the similar field.

He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Previously, atoms were known to be indivisible, but in 1897, j. He zapped atoms with electricity and observed that negatively charged particles were removed! He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. He did the study about electric discharge in high vacuum cathode ray tube. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.. He zapped atoms with electricity and observed that negatively charged particles were removed!

Thomson, a british physicist, conducted the cathode ray experiment. . After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle.

Actually, there were many other scientists who conducted the experiment on the similar field.. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles.

He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Actually, there were many other scientists who conducted the experiment on the similar field. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. He did the study about electric discharge in high vacuum cathode ray tube. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles.. Actually, there were many other scientists who conducted the experiment on the similar field.

He did the study about electric discharge in high vacuum cathode ray tube. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. He zapped atoms with electricity and observed that negatively charged particles were removed!.. Thomson, a british physicist, conducted the cathode ray experiment.

Actually, there were many other scientists who conducted the experiment on the similar field. Thomson, a british physicist, conducted the cathode ray experiment. He zapped atoms with electricity and observed that negatively charged particles were removed! He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. He did the study about electric discharge in high vacuum cathode ray tube.. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles.

Previously, atoms were known to be indivisible, but in 1897, j.. Previously, atoms were known to be indivisible, but in 1897, j. Thomson, a british physicist, conducted the cathode ray experiment. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. He did the study about electric discharge in high vacuum cathode ray tube. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.. Previously, atoms were known to be indivisible, but in 1897, j.

10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Previously, atoms were known to be indivisible, but in 1897, j. Thomson, a british physicist, conducted the cathode ray experiment. He did the study about electric discharge in high vacuum cathode ray tube. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.. He zapped atoms with electricity and observed that negatively charged particles were removed!

He zapped atoms with electricity and observed that negatively charged particles were removed!. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. He zapped atoms with electricity and observed that negatively charged particles were removed! Actually, there were many other scientists who conducted the experiment on the similar field. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. He did the study about electric discharge in high vacuum cathode ray tube. Previously, atoms were known to be indivisible, but in 1897, j. Previously, atoms were known to be indivisible, but in 1897, j.

Previously, atoms were known to be indivisible, but in 1897, j... . Previously, atoms were known to be indivisible, but in 1897, j.

10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application... He zapped atoms with electricity and observed that negatively charged particles were removed! After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. He did the study about electric discharge in high vacuum cathode ray tube. Previously, atoms were known to be indivisible, but in 1897, j. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.

Thomson, a british physicist, conducted the cathode ray experiment. Previously, atoms were known to be indivisible, but in 1897, j. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles.. Actually, there were many other scientists who conducted the experiment on the similar field.

Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. He did the study about electric discharge in high vacuum cathode ray tube. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. He zapped atoms with electricity and observed that negatively charged particles were removed! Thomson, a british physicist, conducted the cathode ray experiment. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Previously, atoms were known to be indivisible, but in 1897, j. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Actually, there were many other scientists who conducted the experiment on the similar field. Previously, atoms were known to be indivisible, but in 1897, j.
.PNG)
Previously, atoms were known to be indivisible, but in 1897, j.. Actually, there were many other scientists who conducted the experiment on the similar field. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. He did the study about electric discharge in high vacuum cathode ray tube.. He zapped atoms with electricity and observed that negatively charged particles were removed!

After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Thomson, a british physicist, conducted the cathode ray experiment. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. He did the study about electric discharge in high vacuum cathode ray tube. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. He zapped atoms with electricity and observed that negatively charged particles were removed!.. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.

10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Thomson, a british physicist, conducted the cathode ray experiment. He did the study about electric discharge in high vacuum cathode ray tube.

Thomson, a british physicist, conducted the cathode ray experiment. He zapped atoms with electricity and observed that negatively charged particles were removed! Actually, there were many other scientists who conducted the experiment on the similar field. Thomson, a british physicist, conducted the cathode ray experiment. Previously, atoms were known to be indivisible, but in 1897, j. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. He did the study about electric discharge in high vacuum cathode ray tube. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Thomson, a british physicist, conducted the cathode ray experiment.

He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. Actually, there were many other scientists who conducted the experiment on the similar field. He zapped atoms with electricity and observed that negatively charged particles were removed! He did the study about electric discharge in high vacuum cathode ray tube. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Previously, atoms were known to be indivisible, but in 1897, j.
He zapped atoms with electricity and observed that negatively charged particles were removed! 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.

Actually, there were many other scientists who conducted the experiment on the similar field... Thomson, a british physicist, conducted the cathode ray experiment. He zapped atoms with electricity and observed that negatively charged particles were removed! He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Actually, there were many other scientists who conducted the experiment on the similar field. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.

Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Thomson, a british physicist, conducted the cathode ray experiment. Actually, there were many other scientists who conducted the experiment on the similar field. He did the study about electric discharge in high vacuum cathode ray tube... After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle.

Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898... Actually, there were many other scientists who conducted the experiment on the similar field. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. He zapped atoms with electricity and observed that negatively charged particles were removed! Previously, atoms were known to be indivisible, but in 1897, j. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. Thomson, a british physicist, conducted the cathode ray experiment. Thomson, a british physicist, conducted the cathode ray experiment.

Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.. He zapped atoms with electricity and observed that negatively charged particles were removed! Thomson, a british physicist, conducted the cathode ray experiment. Previously, atoms were known to be indivisible, but in 1897, j. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Actually, there were many other scientists who conducted the experiment on the similar field. He did the study about electric discharge in high vacuum cathode ray tube.. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle.

Previously, atoms were known to be indivisible, but in 1897, j.. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Thomson, a british physicist, conducted the cathode ray experiment. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. He zapped atoms with electricity and observed that negatively charged particles were removed! Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. He did the study about electric discharge in high vacuum cathode ray tube. Previously, atoms were known to be indivisible, but in 1897, j. Actually, there were many other scientists who conducted the experiment on the similar field.

Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Thomson, a british physicist, conducted the cathode ray experiment. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle.. Previously, atoms were known to be indivisible, but in 1897, j.

10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.. He did the study about electric discharge in high vacuum cathode ray tube. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.

He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Previously, atoms were known to be indivisible, but in 1897, j. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. He did the study about electric discharge in high vacuum cathode ray tube. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. Actually, there were many other scientists who conducted the experiment on the similar field. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Thomson, a british physicist, conducted the cathode ray experiment. He zapped atoms with electricity and observed that negatively charged particles were removed!. Actually, there were many other scientists who conducted the experiment on the similar field.

Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.. He zapped atoms with electricity and observed that negatively charged particles were removed! Thomson, a british physicist, conducted the cathode ray experiment.

Thomson, a british physicist, conducted the cathode ray experiment. Previously, atoms were known to be indivisible, but in 1897, j. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.. Previously, atoms were known to be indivisible, but in 1897, j.

Actually, there were many other scientists who conducted the experiment on the similar field. He did the study about electric discharge in high vacuum cathode ray tube. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. Actually, there were many other scientists who conducted the experiment on the similar field. He zapped atoms with electricity and observed that negatively charged particles were removed!. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.
.PNG)
He zapped atoms with electricity and observed that negatively charged particles were removed! Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. He zapped atoms with electricity and observed that negatively charged particles were removed! 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Actually, there were many other scientists who conducted the experiment on the similar field. Thomson, a british physicist, conducted the cathode ray experiment. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Actually, there were many other scientists who conducted the experiment on the similar field.

He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Previously, atoms were known to be indivisible, but in 1897, j. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Actually, there were many other scientists who conducted the experiment on the similar field. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Thomson, a british physicist, conducted the cathode ray experiment. He zapped atoms with electricity and observed that negatively charged particles were removed! He did the study about electric discharge in high vacuum cathode ray tube. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898... He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles.
Actually, there were many other scientists who conducted the experiment on the similar field. He did the study about electric discharge in high vacuum cathode ray tube. Previously, atoms were known to be indivisible, but in 1897, j. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. Thomson, a british physicist, conducted the cathode ray experiment. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application... 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.

He zapped atoms with electricity and observed that negatively charged particles were removed!.. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. He zapped atoms with electricity and observed that negatively charged particles were removed! Actually, there were many other scientists who conducted the experiment on the similar field. Previously, atoms were known to be indivisible, but in 1897, j. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Thomson, a british physicist, conducted the cathode ray experiment. He did the study about electric discharge in high vacuum cathode ray tube. Thomson, a british physicist, conducted the cathode ray experiment.

He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.. Actually, there were many other scientists who conducted the experiment on the similar field.
He zapped atoms with electricity and observed that negatively charged particles were removed!. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.. Previously, atoms were known to be indivisible, but in 1897, j.

Actually, there were many other scientists who conducted the experiment on the similar field... 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Actually, there were many other scientists who conducted the experiment on the similar field. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. He zapped atoms with electricity and observed that negatively charged particles were removed! He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Previously, atoms were known to be indivisible, but in 1897, j. He zapped atoms with electricity and observed that negatively charged particles were removed!

He zapped atoms with electricity and observed that negatively charged particles were removed! After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.. Previously, atoms were known to be indivisible, but in 1897, j.

Thomson, a british physicist, conducted the cathode ray experiment. Thomson, a british physicist, conducted the cathode ray experiment. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles.

He zapped atoms with electricity and observed that negatively charged particles were removed! He did the study about electric discharge in high vacuum cathode ray tube. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Previously, atoms were known to be indivisible, but in 1897, j. He zapped atoms with electricity and observed that negatively charged particles were removed! Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. Thomson, a british physicist, conducted the cathode ray experiment.. Thomson, a british physicist, conducted the cathode ray experiment.

He did the study about electric discharge in high vacuum cathode ray tube... . After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle.

10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.. Previously, atoms were known to be indivisible, but in 1897, j. Actually, there were many other scientists who conducted the experiment on the similar field. He did the study about electric discharge in high vacuum cathode ray tube. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. Thomson, a british physicist, conducted the cathode ray experiment. He did the study about electric discharge in high vacuum cathode ray tube.

He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. He did the study about electric discharge in high vacuum cathode ray tube. Previously, atoms were known to be indivisible, but in 1897, j. He zapped atoms with electricity and observed that negatively charged particles were removed! Actually, there were many other scientists who conducted the experiment on the similar field. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles.. Thomson, a british physicist, conducted the cathode ray experiment.

He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. He did the study about electric discharge in high vacuum cathode ray tube. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application.

Actually, there were many other scientists who conducted the experiment on the similar field. Actually, there were many other scientists who conducted the experiment on the similar field. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Thomson, a british physicist, conducted the cathode ray experiment. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Previously, atoms were known to be indivisible, but in 1897, j.. Thomson, a british physicist, conducted the cathode ray experiment.

Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898... Actually, there were many other scientists who conducted the experiment on the similar field. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Previously, atoms were known to be indivisible, but in 1897, j. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. He zapped atoms with electricity and observed that negatively charged particles were removed! Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Thomson, a british physicist, conducted the cathode ray experiment. He did the study about electric discharge in high vacuum cathode ray tube. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.

10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Previously, atoms were known to be indivisible, but in 1897, j. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. He zapped atoms with electricity and observed that negatively charged particles were removed! Thomson, a british physicist, conducted the cathode ray experiment. He did the study about electric discharge in high vacuum cathode ray tube. Actually, there were many other scientists who conducted the experiment on the similar field. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle.

Previously, atoms were known to be indivisible, but in 1897, j... . Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898.

He zapped atoms with electricity and observed that negatively charged particles were removed! 10.06.2017 · in 18977, j.j thomson performed several experiments to examine the structure of atom using the cathode ray application. Thomson, a british physicist, conducted the cathode ray experiment. Previously, atoms were known to be indivisible, but in 1897, j. Actually, there were many other scientists who conducted the experiment on the similar field. He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. After the cathode ray tube experiment, thomson gave one of the first atomic models including the newly discovered particle. Eine genauere modellvorstellung über die ladungsstruktur der atome entwickelte joseph john thomson um 1898. He zapped atoms with electricity and observed that negatively charged particles were removed! He did the study about electric discharge in high vacuum cathode ray tube. Actually, there were many other scientists who conducted the experiment on the similar field.